What are the side effects? Is it safe? Doctors answer common COVID vaccine questions
Published December 15, 2020 at 7:17 pm
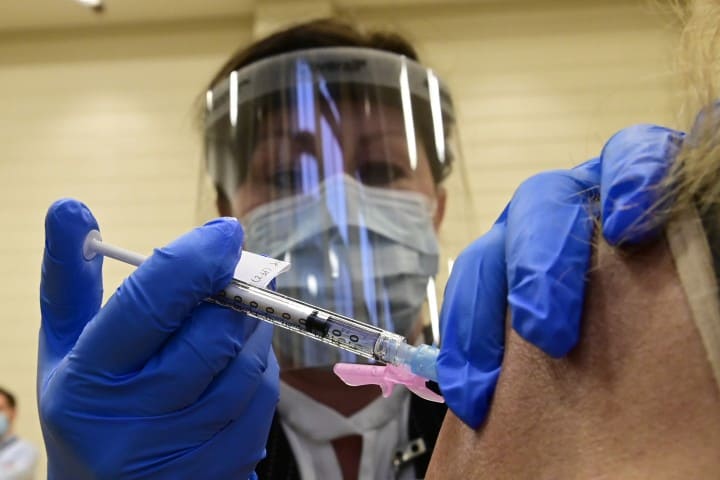
TORONTO —
As the first batch of vaccinations target groups most at risk of COVID-19 exposure and illness, many Canadians have questions about how the broader rollout will look when it’s their turn for a shot.
The Ontario Medical Association convened a panel of experts to tackle the most common questions they’ve heard from patients and posted their advice on social media. Here’s a look at some of their answers, which have been edited for length and clarity:
Q: WHAT WILL I FEEL AFTER THE VACCINE? WHAT ARE THE SIDE EFFECTS?
Pediatric immunologist Dr. Zainab Abdurrahman explains that vaccines include little bits of a pathogen that trick your body into thinking it is getting infected. That generates an immune response your body will remember if it ever encounters the actual virus. As a result, your body will go through the natural steps of fighting an infection and so you may feel symptoms of illness including fever, localized pain and swelling at the injection site, and general unwellness.
“You can expect to feel similar to how people feel with the influenza vaccine, similar to how you might feel with any of the other vaccines you get,” says Abdurrahman.
“These are actually just signs of your immune system working.”
Q: I HEARD THAT PEOPLE WHO HAVE EXPERIENCED ANAPHYLAXIS SHOULD NOT TAKE THE VACCINE. WHO ELSE SHOULD NOT TAKE THE VACCINE?
Zainab notes two people in the United Kingdom had anaphylaxis-like reactions after receiving their shot and recovered quickly after receiving epinephrine. However, she says people with allergies were included in the trials and a review of clinical data showed no increased risk of anaphylaxis. She adds that the U.S. Food and Drug Administration, Health Canada and Canadian Society of Allergy and Clinical Immunology say the only people who should avoid the Pfizer vaccine are those who’ve had an allergic reaction to the first dose of the two-dose regime, or those allergic to one of the components. Zainab notes the component polyethylene glycol seems to drive some reactions, but says this is a very rare allergy.
“If you have this allergy, you’re well known to an allergist by this point. Polyethylene glycol is actually in a lot of medications which we use routinely, most commonly being Tylenol,” she says.
The National Advisory Committee on Immunization recommends those who are pregnant or breastfeeding should also avoid the vaccine, and pediatric allergist Dr. Mariam Hanna says there isn’t enough data on this group to know how they would respond. Still, she allows there is room for some to consider vaccination in consultation with their doctor.
“We exercise extreme caution but we always have to balance between risks and benefits — risk of what we know (against) risk of what we don’t know — and come up with a shared decision towards that,” says Hanna.
“The more information that we learn, the more that this becomes something that we are more comfortable in offering all pregnant people and all breastfeeding families.”
Q: IS THE COVID-19 VACCINE SAFE?
Hanna says vaccine safety is heavily scrutinized in clinical trials, and that continues when the vaccine is rolled out into the real world. While mild side effects are expected, experts on the local, provincial, national and international level are on the lookout for adverse reactions that could suggest the need for further evaluation.
“We know it’s safe. We continue to gather data that will ensure that it’s safe,” says Hanna.
“The real-world data is probably the most exciting part to look at as vaccines get implemented, so that when it’s your turn to get the vaccine the safety data is not only 44,000 (trial) patients, it’s in the millions by that point.”
Q: WHY ARE CHILDREN NOT ELIGIBLE FOR THIS VACCINE? HOW WILL WE ACHIEVE HERD IMMUNITY IF WE CANNOT VACCINATE CHILDREN FOR ANOTHER YEAR?
Hanna says the current vaccine is approved for kids age 16 and older, and that ongoing trials are looking at those aged 12 to 15, and younger than 12. But it’s not possible to simply extrapolate trial data involving adults to children.
“A child’s immune system behaves a little bit differently throughout their childhood and that’s why we start looking at different age groups,” says Hanna.
“We need to have immunity in children, but we need safety data, efficacy data and that is coming… We need to continue to keep our guard up during this transition time. Kids aren’t included in the Phase 1 rollout, maybe in Phase 2, but probably Phase 3 will definitely include kids.”
Q: HOW SOON AFTER GETTING THE VACCINE CAN WE RETURN TO LIFE BEFORE COVID-19?
The Pfizer vaccine has very high efficacy, which bodes well for its real-world success, says Dr. Vinita Dubey, associate medical officer of health for Toronto Public Health. But she warns it will take time to see a significant impact on the pandemic.
Dubey says that when the polio vaccine emerged in the 1950s, spikes of polio cases continued for years.
“You can certainly get a dramatic decline from vaccines but it still takes time, it’s not overnight. It still can take months or even years,” says Dubey. “Part of that is because we have to get everyone vaccinated and that’s going to take time as well.”
Until then, Dubey says the public will still have to take COVID-19 precautions and wear masks, physical distance, stay home if sick and screen school children.
Cassandra Szklarski, The Canadian Press
insauga's Editorial Standards and Policies advertising





