Counterfeit Botox and IKEA products among items recalled in Mississauga, Brampton and area
Published September 11, 2022 at 4:25 pm

A common household item, window blinds, and a not so common beauty product, Botox, are both facing recalls this week in Mississauga, Brampton, and surrounding areas.
Some blinds from the Swedish home goods giant, IKEA did not make it past Canadian Corded Window Coverings Regulations. And Health Canada has seized a counterfeit Botox product from a spa in Woodbridge.
See below for more details and information on each specific recall.
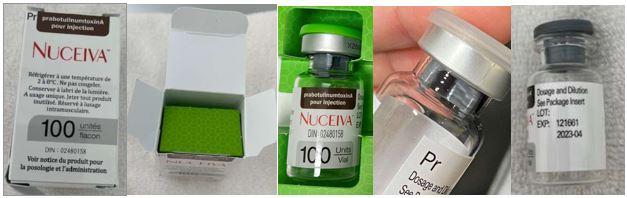
Risk: Health Canada seized counterfeit Nuceiva (Prabotulinumtoxin A) injectable Botox from a spa in Woodbridge. Nuceiva is a botulinum toxin type A prescription drug that is administered by injection for the temporary improvement of facial wrinkling in adults 18 years of age and older.
New You Spa is located at 60 Winges Rd. in Woodbridge, near Brampton. Their services range from advanced cosmetic injectables, to acne solutions and laser hair removal.
Health Canada has confirmed with the manufacturer, Evolus Incorporated, that the seized products are counterfeit. The counterfeit Nuceiva is labelled with lot 121661 and expiry 2023-04. Evolus Inc. confirmed there is an authorized lot with the same number, but the lot expired in 2022-04.
Packaging colour is another difference between the genuine product and the counterfeit product. The counterfeit carton has a green inlay while the authentic Nuceiva carton is white. The counterfeit carton is also missing the manufacturer (Evolus Inc.) and distributor (Clarion Medical Technologies Inc.) information.
Botulinum toxin type A (Botox) is used for cosmetic purposes to treat facial wrinkling. Potential risks associated with injecting an unauthorized Botulinum toxin type A product can range from mild local paralysis to death.
Unauthorized injectable health products for cosmetic purposes carry significant risk because of the potential for infection, scarring and poor outcomes.
What you should do: Do not use this counterfeit product. Consult with a health care professional if you think you may have been administered this product at this location and you have health concerns. Report any health product-related adverse reactions or complaints to Health Canada.
IKEA TRIPPEVALS and HOPPVALS cellular blinds





Risk: IKEA TRIPPEVALS and HOPPVALS cellular blinds are recalled due to the potential choking hazard for children.
Health Canada has determined that the recalled blinds do not meet the Corded Window Coverings Regulations. The design of the product does not properly address the hazards of small parts which can present a choking hazard to young children.
The HOPPVALS blinds are sold in three colours, white, grey, and blue. The TRIPPEVALS blinds are slightly larger and sold in light grey and white.
IKEA reported that 127,857 units of the affected products were sold in Canada from May 2021 to July 2022.
Residents should note that the Canada Consumer Product Safety Act prohibits recalled products from being redistributed, sold or even given away in Canada.
What you should do: Immediately stop using the recalled IKEA TRIPPEVALS and HOPPVALS cellular blinds and contact IKEA for a repair/replacement kit. Customers who do not wish to order a repair kit may return the product to an IKEA store for a refund.
Chicology cordless 1-inch vinyl mini blinds


Risk: Chicology cordless 1-inch vinyl mini blinds are recalled due to strangulation and choking hazards to children. This recall involves Chicology mini blinds UPC number 8 12030 03236 0, model VNBGW4348. The UPC number can be found on the packaging material. The product itself does not have a label displaying UPC or model number.
Health Canada has determined that the recalled blinds do not meet the Corded Window Coverings Regulations and pose a strangulation and choking hazard. The various configurations of the products can create loops exceeding 44cm.
Young children may put looped cords around their neck causing a strangulation and entanglement hazard. Health Canada has also determined that the bottom rail end caps may detach, creating a small part and posing a choking hazard to young children.
The company reported that 687 units of the affected product were sold in Canada from October 2019 to July 2022.
What you should do: Consumers should immediately stop using the recalled Chicology mini blinds and contact the company to arrange a refund. Contact Chicology by email at [email protected].
INsauga's Editorial Standards and Policies








